Abstract
Background: Leukemic relapse remains the major cause of treatment failure in hematopoietic stem cell transplant (HSCT) recipients. While the infusion of donor lymphocytes to prevent and treat relapse has been clinically implemented this strategy does not provide durable remissions and carries the risk of life-threatening graft-versus-host disease (GVHD). More recently the adoptive transfer of T cells that have been engineered to express CD19-targeted chimeric antigen receptors (CARs), has shown potent anti-leukemic activity in HSCT recipients with recurrent disease. However, disease relapse with the emergence of CD19 negative tumors is an emerging clinical issue post-administration of these mono-targeted T cells. To overcome these limitations, we developed a protocol for the generation of donor-derived T cell lines that simultaneously targeted a range of tumor associated antigens (multiTAAs) that are frequently expressed by B- and T-cell ALL including PRAME, WT1 and Survivin for adoptive transfer to high risk recipients transplanted for ALL.
Methods/Results: We were consistently able to generate donor-derived multiTAA-specific T cells by culturing PBMCs in the presence of a Th1-polarizing/pro-proliferative cytokine cocktail, using autologous DCs as APCs and loading them with pepmixes (15 mer peptides overlapping by 11 amino acids) spanning all 3 target antigens. The use of whole antigen increases the range of patient HLA polymorphisms that can be exploited beyond those matched to single peptides, while targeting multiple antigens simultaneously reduces the risk of tumor immune evasion. To date, we have generated 14 clinical grade multiTAA-specific T cell lines comprising CD3+ T cells (mean 94±9%) with a mixture of CD4+ (mean 21±28%) and CD8+ (mean 52±24 %) cells, which expressed central [CD45RO+/CD62L+: 14±9%] and effector memory markers [CD45RO+/CD62L-: 80±11%] associated with long term in vivo persistence. The expanded lines recognized the targeted antigens WT1, PRAME and Survivin by IFNg ELIspot with activity against >1 targeted antigens in all cases. None of the lines reacted against non-malignant patient-derived cells (4±3% specific lysis; E: T 20:1) - a study release criterion.
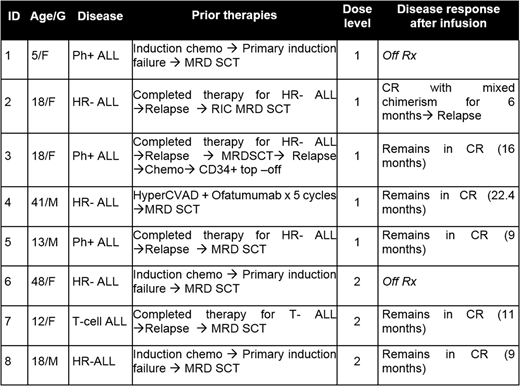
Thus far we have treated 8 high risk ALL patients with donor derived TAA T cells post-transplant to prevent disease relapse (Table 1). Infusions were well tolerated with no dose-limiting toxicity, GVHD, CRS or other adverse events. Two patients were not evaluable per study criteria as they received >0.5mg/kg of steroids within 4 weeks of infusion and were replaced. Five of the 6 remaining patients infused remain in CR a median of 11.2 months post-infusion (range 9-22 months). We detected the expansion of tumor-reactive T cells in patient peripheral blood post-infusion against both targeted (WT1, Survivin, PRAME) and non-targeted antigens (SSX2, MAGE-A4, -A1, -A2B, -C1, MART1, AFP and NYESO1) reflecting epitope and antigen spreading. The single patient who relapsed showed no evidence of tumor-directed T cell expansion despite receiving 3 additional infusions at 4 week intervals.
Conclusion: In summary, infusion of donor multi-TAA-specific T cells to patients with ALL post allogeneic HSCT is feasible, safe and as evidenced by expansion and antigen spreading in patients, may contribute to disease control. This strategy may present a promising addition to current immunotherapeutic approaches for prophylaxis for leukemic relapse in HSCT recipients.
Vera:Marker: Equity Ownership. Heslop:Marker: Equity Ownership; Cytosen: Membership on an entity's Board of Directors or advisory committees; Cell Medica: Research Funding; Gilead Biosciences: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Research Funding; Viracyte: Equity Ownership. Leen:Marker: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal